The histology and histochemistry of man : a treatise on the elements of composition and structure of the human body / by Heinrich Frey ... Translated from the fourth German edition, by Arthur E.J. Barker ... and revised by the author. With six hundred and eight engravings on wood.
- Frey, Heinrich, 1822-1890.
- Date:
- 1874
Licence: Public Domain Mark
Credit: The histology and histochemistry of man : a treatise on the elements of composition and structure of the human body / by Heinrich Frey ... Translated from the fourth German edition, by Arthur E.J. Barker ... and revised by the author. With six hundred and eight engravings on wood. Source: Wellcome Collection.
Provider: This material has been provided by King’s College London. The original may be consulted at King’s College London.
53/698 (page 41)
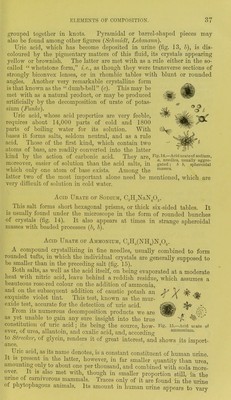
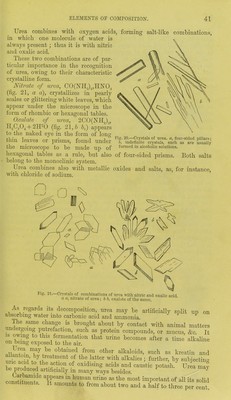
![Urea combines with oxygen acids, forming salt-like combinations, in which one molecule of water is always present ; thus it is with nitric and oxalic acid. These two combinations are of par- ticular importance in the recognition of urea, owing to their characteristic crystalline form. Nitrate of urea, CO(NIL).,,HXO:J (fig. 21, a a), crystallizes in pearly scales or glittering white leaves, which appear under the microscope in the form of rhombic or hexagonal tables. Oxalate of urea, 2CO(NH,)„ H2C,04 + 2H20 (fig. 21, b b,) appears to the naked eye in the form of long thin leaves or prisms, found under the microscope to be made up of hexagonal tables as a rule, but also of four-sided prisms. Both salts belong to the monoclinic system. Ijrea combines also with metallic oxides and salts, as, for instance, with chloride of sodium. Fig. 20.—Crystals of urea, a, four-sided pillars; 6, indefinite crystals, such as are usually formed in alcoholic solutions. As regards its decomposition, urea may be artificially split up on absorbing water into carbonic acid and ammonia. The same change is brought about by contact with animal matters undergoing putrefaction, such as protein compounds, or mucus, &c. It is owing to this fermentation that urine becomes after a time alkaline on being exposed to the air. n?ay ke obtained from other alkaloids, such as kreatin and °/ the Iatter with alkalies; further, by subjecting bp Lnrld t0i th?-oCtlnn °f oxld]SlnS acids and caustic potash. Urea may be produced artificially in many ways besides. 3 arbamide appears in human urine as the most important of all its solid constituents. It amounts to from about two and a half to three per cent.](https://iiif.wellcomecollection.org/image/b21310178_0053.jp2/full/800%2C/0/default.jpg)