Handbook of the polariscope and its pracitcal applications / adapted from the German editon of H. Landolt, by D.C. Robb and V.H. Veley.
- Landolt, H. (Hans), 1831-1910.
- Date:
- 1882
Licence: Public Domain Mark
Credit: Handbook of the polariscope and its pracitcal applications / adapted from the German editon of H. Landolt, by D.C. Robb and V.H. Veley. Source: Wellcome Collection.
41/320 (page 21)
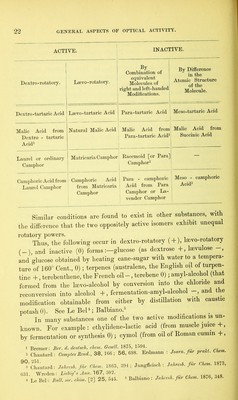
![either of two structurally opposed (or enantiomorphous) shapes. Molecules of the former class possess symmetry of structure; those of the latter class have their atoms disposed asymmetrically, and accordingly exhibit optical activity. In 1848, Pasteur1 * made the important discovery that inactive para-tartaric acid is separable into right-rotating and left-rotating tartaric acids; and the sodium- ammonium salts of these two acids are distinguishable from each other by the presence of dextro-hemihedric and laevo-hemihedric planes respectively. Moreover, these salts retain their opposite characters in solution, by exhibiting opposite rotatory powers. Hence we may suppose that the property of asymmetrical structure of opposite kinds, such as we have seen in crystals, may occur in molecules also, and the precise nature of the arrangement of the atoms, or rather atom-groups, may reasonably be assumed to be here also of a helical kind. Whether the phenomenon of circular double- refraction, as exhibited by crystals, occurs also in active liquids is still an undecided point, several experiments made by Dove3 on sugar solutions and on oil of turpentine having led to no conclusive result. Hence, according to Pasteur’s3 views, the different optical modifi- cations of tartaric acid may be explained on the supposition that in dextro-tartaric acid the atoms which go to form the molecule are grouped in right-handed helices, whilst in lsevo-tartaric acid they are grouped in helices, equal in size, but left-handed in direction : and hence, too, the inactivity of racemic (para-tartaric) acid on the ground of its being formed by the union of equal molecules of the two former modifications. But besides these, other forms are well known, optically inactive, but not separable into the two optically active acids. To explain the existence of these, some other assump- tion is necessary, either that the helical structure is in their case abolished (untwisted), as Pasteur suggests, or that their inactivity arises from compensation within the molecule which is composed of two atom-groups possessing opposite rotatory powers. As to chemical structure, however—that is, the distribution of affinities between the atoms—they do not differ from the other isomeric acids. Analogous optical modifications have been observed in a few other substances, which have been brought together in the table on page 22. 1 Pasteur: Ann. CMm. Phys. [3] 24, 442; 28, 56; 38, 437. 3 Dove: Pogg. Ann. 110, 290. 3 Pasteur: Eecherches, &c. p. 38.](https://iiif.wellcomecollection.org/image/b28125952_0041.jp2/full/800%2C/0/default.jpg)