Blood : a study in general physiology / by Lawrence J. Henderson.
- Henderson, Lawrence J. (Lawrence Joseph), 1878-1942.
- Date:
- 1928
Licence: In copyright
Credit: Blood : a study in general physiology / by Lawrence J. Henderson. Source: Wellcome Collection.
87/434 (page 61)
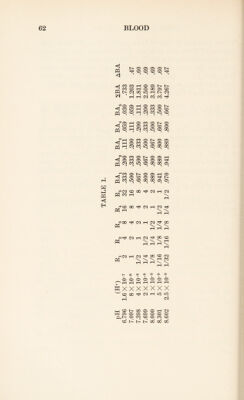
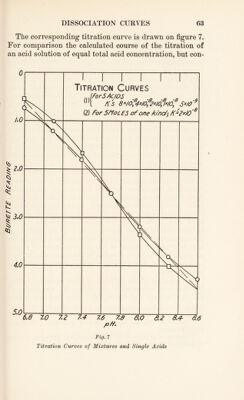
![These radicals therefore take part in the acid-base equi¬ librium of blood and compete with carbonic acid for base. In approaching the study of this question, we may first consider the case of a number of simple monobasic acids, HAi, HAn, . . . HAn, all of which obey the law of acid- base equilibrium in blood discussed in the last chapter. Here we shall have [H+] = k\ [HA,] [BA:] [HAn]_ 2 [BAn] “ , [HAn] N [BAN] * Let there be five such acids, all present in a solution in equal concentration, so as to correspond with the case of a polybasic protein, and let the k' values be 8 X 10~8, 4 X 10~8, 2 X 10'8, 1 X 10-8, and 5 X 10-9. Then the titra¬ tion curve for the solution may be calculated. The results of this calculation are given in table 1, which includes values of pH, of [H+], of R = [HA] [BA] and of [BA] for each acid and of 2[BA], which is the burette reading for each stage of the titration.](https://iiif.wellcomecollection.org/image/b29928771_0087.jp2/full/800%2C/0/default.jpg)