Blood : a study in general physiology / by Lawrence J. Henderson.
- Henderson, Lawrence J. (Lawrence Joseph), 1878-1942.
- Date:
- 1928
Licence: In copyright
Credit: Blood : a study in general physiology / by Lawrence J. Henderson. Source: Wellcome Collection.
85/434 (page 59)
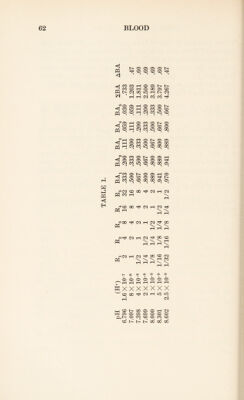
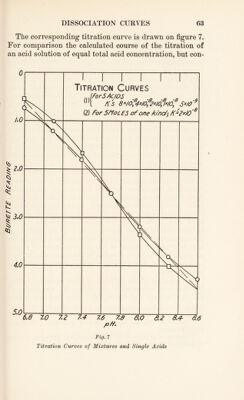
![acids than the ion H2P04~, and to further complications owing to the fact that several acid radicals of the blood proteins are simultaneously engaged in the process. In sum we have now reached the conclusion that the study of the simple equation, [H+] [H2CQ3] [BHC03]’ which gives in a close approximation the facts concerning the state of carbonic acid and its salts in blood, is capable of explaining many features of general and respiratory physiology. Among these are the homogeneous and het¬ erogeneous buffer action of carbonic acid, the physiologi¬ cal regulation of the alkalinity of blood through the ac¬ tivity of lungs and kidneys, and some of the features of acidosis. When the same approximation is also applied to other weak acids, we are enabled to understand the na¬ ture of the process by which carbonic acid is absorbed by blood in the tissues and given off in the lungs. This de¬ scription of the facts, a quantitative one, also enables us to estimate the efficiency of carbonic acid in some of these physiological functions, and to draw the conclusions that for such purposes its properties are, on the whole, unsur¬ passed and almost unequalled by any other substance.49 Nevertheless it would be an error to suppose that we have already exhaustively analyzed all features of these physiological functions. Not only are such activities far more intricate in their ramifications than the present dis¬ cussion suggests, but in the conditions of equilibrium be¬ tween red cells and plasma, and of the peculiar activity of hemoglobin there may be recognized laws of which we have not yet taken account. Moreover the specific proper¬ ties of the acid-base equilibrium of the proteins also have to be studied if we are to arrive at an exact estimate of the conditions in blood. Accordingly we must now pass on to the proteins of blood and to the dissociation curves of the physiologists. 49 L. J. Henderson, American Journal of Physiology, XXI, 173 (1908).](https://iiif.wellcomecollection.org/image/b29928771_0085.jp2/full/800%2C/0/default.jpg)